


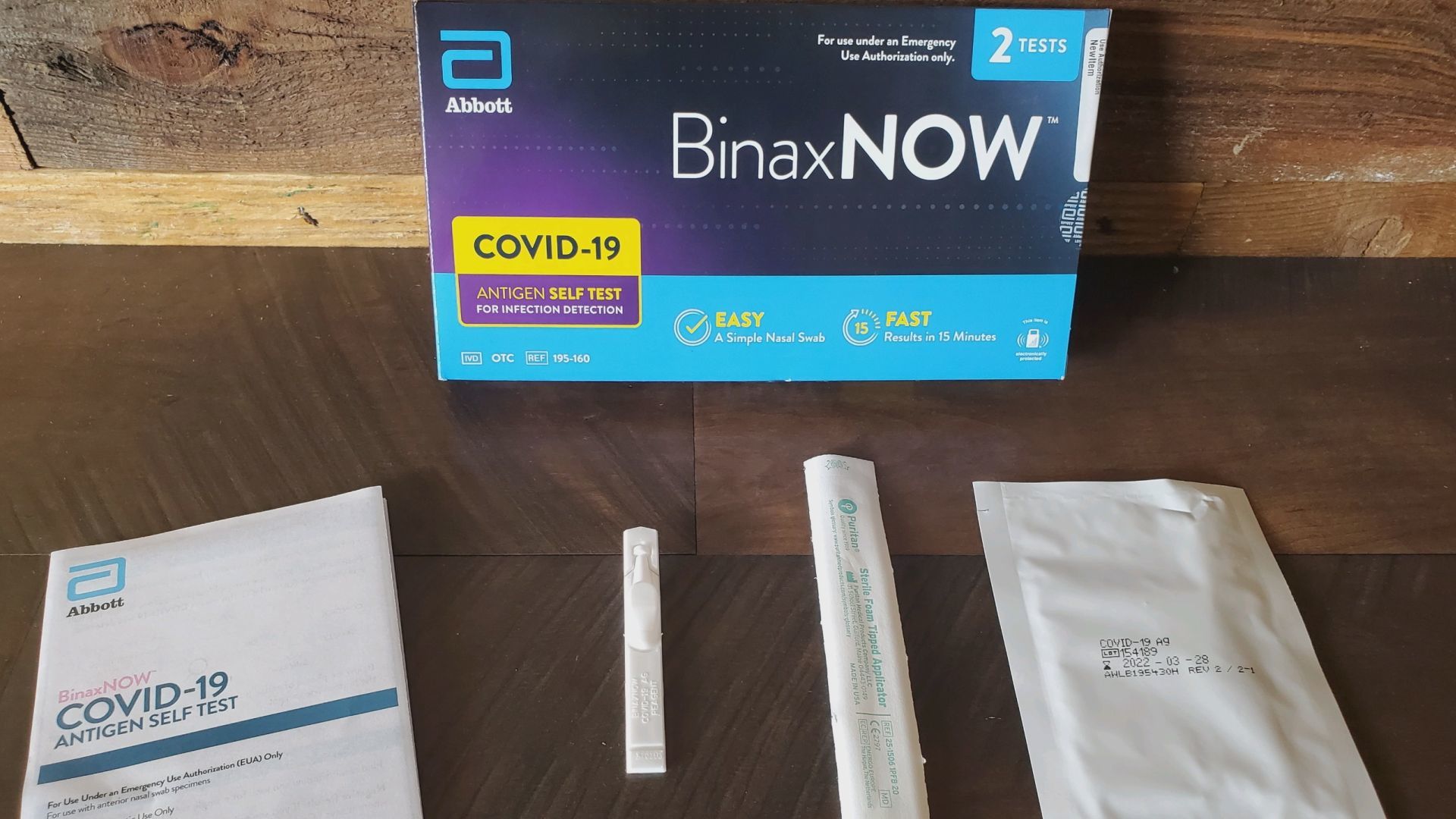


Abbott BinaxNOW At Home Antigen Self Test in Canada
Product Details:
Abbott BinaxNOW At Home Antigen Self Test in Canada Price And Quantity
- 1 Box
- 1.2-1.5 USD ($)/Box
- 1.20 - 1.50 USD ($)/Box
Abbott BinaxNOW At Home Antigen Self Test in Canada Trade Information
- USA
- 3 Box Per Day
- 5-15 Days
- Australia Western Europe Central America Middle East South America Asia Eastern Europe North America Africa
- Abbott BinaxNOW At Home Antigen Self Test in Canada.FDA EUA CE ISO GMP SFC ETC
Product Description
The BinaxNOW COVID-19 Antigen Self Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. Abbott BinaxNOW At Home Antigen Self Test in Canada This test is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 15 years or older or adult collected anterior nasal swab samples from individuals aged two years or older.
The BinaxNOW COVID-19 Ag tests have not been FDA cleared or approved. They have been authorized by the FDA under an emergency use authorization. Abbott BinaxNOW At Home Antigen Self Test in Canada The tests have been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens, and are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese








